Error propagation
Contents
Error propagation¶
How error propagates through calculations and anlysis¶
Imagine you have three variables:
\(a\), \(b\), \(c\)
Each of those variables has a measure of uncertainty:
\(\sigma_a,\sigma_b,\sigma_c\)
Some calculated variable \(y = f(a,b,c)\)
What is the uncertainty of y when measurements are dependant/inter-related?
\(\sigma_y^2 \approx \sigma_a^2 \left(\frac{\partial y}{\partial a}\right)^2 +\sigma_b^2 \left(\frac{\partial y}{\partial b}\right)^2 + \sigma_c^2\left(\frac{\partial y}{\partial c}\right)^2 + 2\sigma_{ab} ^2 \left(\frac{\partial y}{\partial a}\right)\left(\frac{\partial y}{\partial b}\right) + 2\sigma_{bc}^2 \left(\frac{\partial y}{\partial b}\right)\left(\frac{\partial y}{\partial c}\right) + 2\sigma_{ac}^2 \left(\frac{\partial y}{\partial a}\right) \left(\frac{\partial y}{\partial c}\right)\)
This equation shows that the uncertainty in the calculated variable \(y\) depends on:
The uncertainty in the measurement variables (\(\sigma_a,\sigma_b,\sigma_c\))
The covariance among measurment variables (\(\sigma_{ab}^2,\sigma_{bc}^2,\sigma_{ac}^2\))
The relationship between magnitudes of the measurement variables and calculated variable (\(\partial y\)/\(\partial a\), \(\partial y\)/\(\partial b\), \(\partial y\)/\(\partial c\))
Special cases
¶
Rules for error propagation in common operations can be derived from the equation above. If there all measurement variables are independent, then the terms involving covariance become zero. The examples listed below assume no correlation between measurement variables.
A more complete table, including covariance terms, can be found on Wikipedia: https://en.wikipedia.org/wiki/Propagation_of_uncertainty#Example_formulas
Addition, subtractions¶
\(y = a + b - c\)
error:
\(\sigma_y ^2 = \sigma_a ^2 + \sigma_b ^2 + \sigma_c ^2\)
Multiplication, division¶
\(y = \frac{ab}{c}\)
relative error:
\((\frac{\sigma_y }{y})^2 =(\frac{\sigma_a }{a})^2 + (\frac{\sigma_b }{b})^2 + (\frac{\sigma_c }{c})^2\)
Multiplying by an exact number¶
\(y=ma\)
error:
\((\sigma_y) = m(\sigma_a)\)
relative error:
\((\frac{\sigma_y}{y})^2 = (\frac{\sigma_a}{a})^2\)
Python uncertainties package¶
The uncertainties package in Python makes it easy to keep track of uncertainties in complicated equations and account for correlations, which are ignored in the special cases above.
Example¶
Example: Measure diameter of a sphere:
relative error = 10%
Error in calculating volume?
\(V = \frac{4}{3} \pi r^3\)
This problem can be approached by breaking it into several parts. First, we have the relative error in measuring the diameter, what is the error in calculating the radius,
\(r = \frac{1}{2}d\)?
The absolute error changes when calculating the radius,
but the relative error does not,
Similarly, the relative error of the volume, \(V\), is equal to the relative error in calculating \(r^3\),
The error in \(r^3\) can be found by using the rule for powers,
which is equivalent to
This result can be checked with the uncertainties package.
from uncertainties import ufloat
import numpy as np
d = ufloat(10,0.1)
V = (4/3)*np.pi*(0.5*d)**3
V.std_dev/V.nominal_value
0.029999999999999995
Example: Marine Carbonate Chemistry
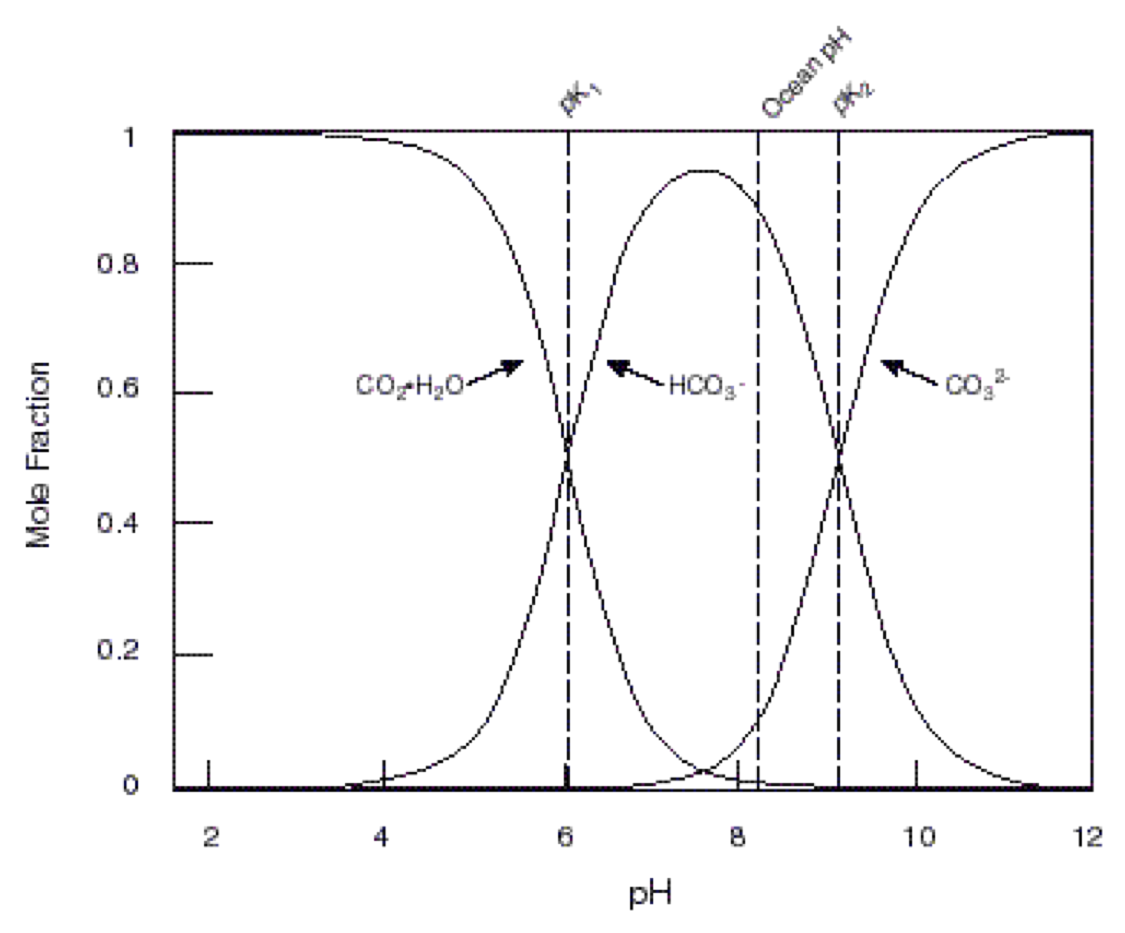
Relative concentrations of dissolved CO2 and carbonate ions in seawater are determined by pH pf seawater and dissociation constants \(K_1'\) and \(K_2'\)
DIC = dissolved inorganic carbon
DIC = [CO2] + [HCO3-] + [CO3-2]
\(CO_2 + H_2 O \iff HCO_{3}^- + H^+\)
\(k_1'= \frac{[HCO3-][H^+]} {[CO_2]}\)
\(HCO_3 ^- \iff CO_3 ^2- +H^+\)
\(k_2' =\frac{ [CO3- ^2-][H^+]}{[HCO_3 ^-]}\)
partial pressure of CO2
\(pCO2 = \frac{[CO2]}{K_{h,CO_2}}\) Henry’s law constant = 3.24X10\(^-2\) \(\frac{mol}{kg atm}\) at 20 degrees C, s=35 PSU
\(pK_1' = 5.847\)
\(pK_2' = 8.966\)
\(pH = -\log_{10}{[H^+]} \to [H^+] = 10^{-pH}\)
\(pK_1' = -\log_{10}{K_1'} \to K_1' = 10^{-pK}\)
We can measure pCO_2 and pH well, but not DIC, but not alkalinity
\([CO_2] = \frac{DIC} {1 + \frac{k_1'}{H^+} + \frac{k_1' k_2'}{[H^+]^2}}\)
Analytical error in pH = 0.0020
Analytical error in DIC = umol kg-1
What is the \(\sigma_{H^+}\)?
\(\sigma_{H^+} = 2.303[H^+]\sigma_{pH}\)
To get the order of magnitude assume a pH around 8.2 (typical for seawater)
\([H^+] \approx 10^{-8.2}\)
What is the error in calculating the partial pressure of CO2 in seawater from these measurements?